The AU-CD beam line on ASTRID2
Synchrotron Radiation Circular Dichroism.
Circular Dichroism (CD) spectroscopy measures the difference in absorption between left and right handed circularly polarized light in chiral molecules. It is an established biophysical method probing the secondary structure (e.g. helices, beta-sheets, turns etc.) of peptides, proteins and nucleic acids which have distinct CD bands in the far-UV and VUV.
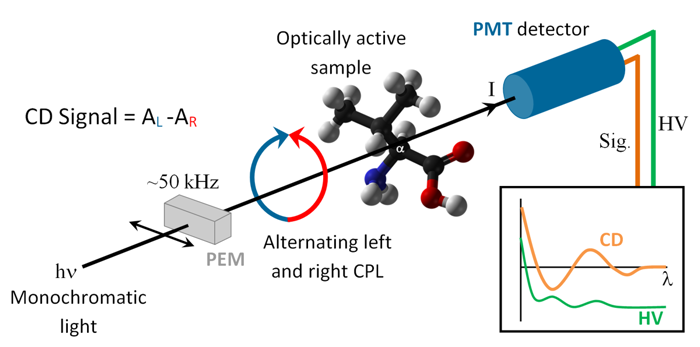
The principle behind CD spectroscopy. Monochromatised light from CD1 is passed through a Photo Elastic Modulator (PEM) which converts the linear polarised light
into alternating left and right handed polarised light. The two polarisations are differently absorbed, and the difference in absorption is detected with a
Photo Multiplier Tube (PMT)
Secondary structures give rise to characteristic far UV and VUV CD spectra. A CD spectrum can be considered to arise from the weighted summed components of secondary structure that comprise a protein. Studies have shown that the information content in these data (i.e. how many different types of secondary structures can accurately be distinguished) is highly dependent on the wavelength range of the spectrum. Using data between 200 and 260 nm, the information content is two, rising to three if data down to 190 nm are used. If the data extend down to 178 nm, the value is five, and by taking spectra to 160 nm the information content rises to about eight. Thus the additional wavelength range offered by SRCD greatly enhances the number of secondary structure types that can be distinguished in a CD spectrum.
Last Modified 02 July 2021